
The Problem
Whether or not to Biopsy – Being on the fence about next steps in the diagnostic journey can create anxiety and fear in you and your patient and potentially stalling next steps.
And for many pulmonary nodules you’re not even sure a biopsy is the right next step; the uncertainty in those cases can be just as challenging—stalling decisions and putting both you and your patient in a holding pattern.
Lung Cancer Statistics:
- Up to 13% of surgical lung resections from lung cancer screening programs are benign. (https://pmc.ncbi.nlm.nih.gov/articles/PMC10730375/)
- Early detection improves survival rates significantly. (https://shorturl.at/CywEU)
- CTB and RAB are revolutionizing the diagnosis of lung cancer, providing diagnostic yields above 80- 90%. (https://pmc.ncbi.nlm.nih.gov/articles/PMC10888048)
How LungLifeAI Works:
The Science Behind the Innovation
LungLifeAI is powered by a proprietary 4-color fluorescence in situ hybridization (FISH) assay that identifies Circulating Genetically Abnormal Cells (CGACs) in a standard blood sample. These CGACs exhibit specific chromosomal abnormalities frequently found in lung cancer cells and focus on whole-cell abnormalities that are reliably detected even in small tumors, allowing early detection of malignant transformation when imaging results are inconclusive.
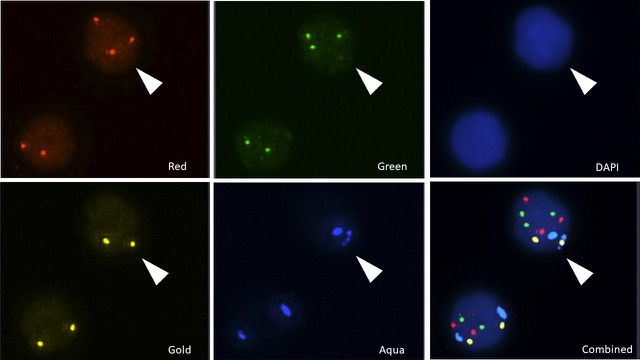
FIGURE B: CGAC IDENTIFICATION
- Illustrates the clear difference between normal and genetically abnormal cells under FISH imaging.
- Normal cells show two signals per probe, while CGACs show multiple or missing signals, indicating genomic instability.
Utilizing the Power of AI
The LungLifeAI test uses a locked and validated machine learning-based image analysis algorithm to automatically identify Circulating Genetically Abnormal Cells (CGACs) from fluorescent imaging. To develop this classifier, this algorithm scanned thousands of cells, detecting abnormal chromosomal signal patterns, and applies predefined decision rules to classify CGACs with high precision. It minimizes human error and interobserver variability, making the test more reproducible and scalable for clinical use

Clinical Validation of LungLifeAI
A pivotal study published in BMC Pulmonary Medicine evaluated the efficacy of LungLifeAI in predicting lung cancer among individuals with IPNs. The study enrolled 151 participants scheduled for biopsy across two renowned institutions: Mount Sinai Hospital and MD Anderson Cancer Center. The primary objective was to assess the correlation between LungLifeAI results and biopsy-confirmed diagnosis.
Key Benefits:
Implications for Clinical Practice:
Clinical Highlights
American Lung Association. State of Lung Cancer – Key Findings.
Published November 14, 2023. Accessed April 4, 2025.
https://www.lung.org/research/state-of-lung-cancer/key-findings

